
|
|

|
||
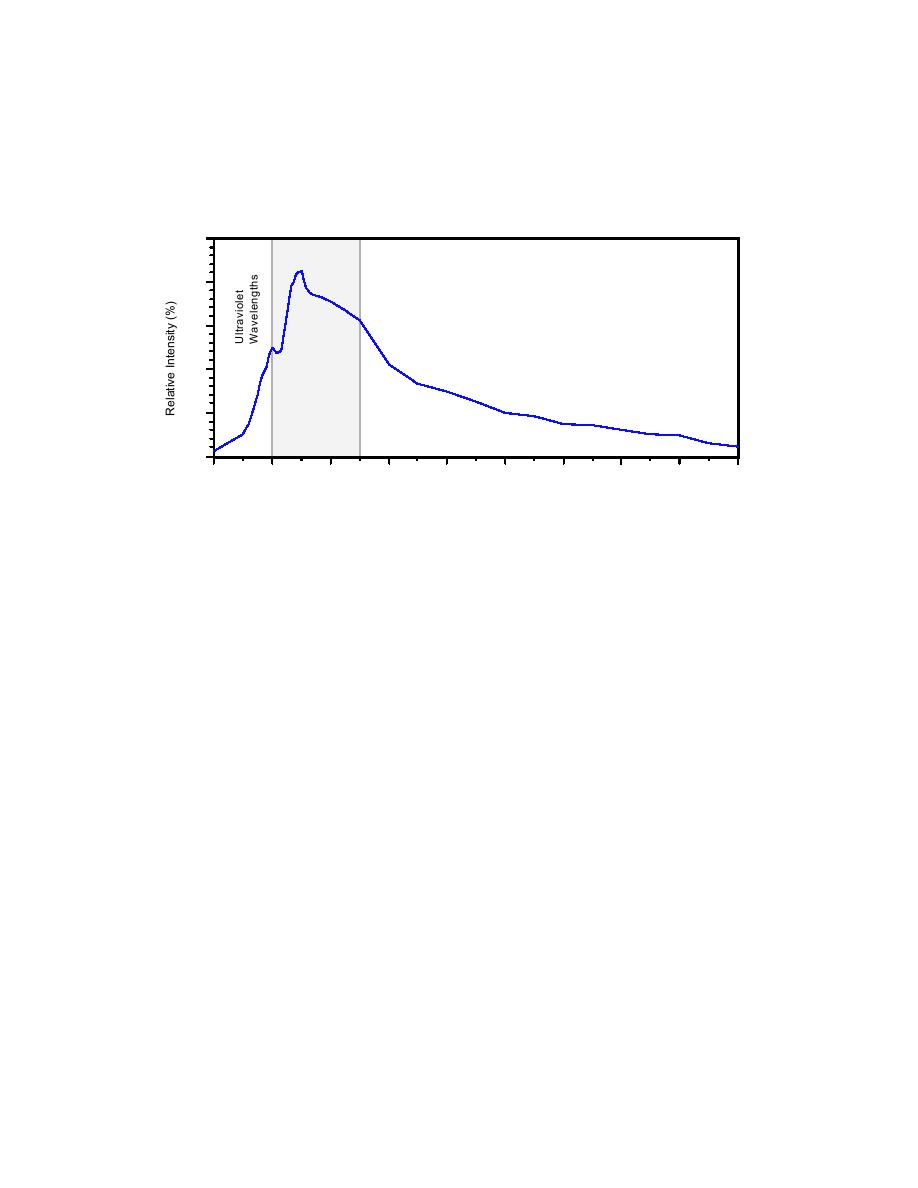 energy for the solar spectrum. At the edge of our atmosphere peak energy for the solar spectrum
occurs at the blue edge of the visible range at approximately 380 nm. At the surface of the earth,
because of the atmospheric intervention, the peak energy occurs between 500 and 600 nm,
approximately centered in the visible range (Goldman and Horne 1983).
Blue
Green
Red
100
80
Near Infrared
Wavelengths
60
Visible
40
Far Infrared
Wavelengths
Wavelengths
20
0
200
400
600
800
1000
1200
1400
1600
1800
2000
Wavelength of Solar Radiation (nm)
Figure 1.2.2 Wavelengths of Solar Radiation versus Relative Intensity
1.2.5.4 Physical Properties of Water
Water is one of the most important substances for life. Most of the mass of organisms is water
and water is necessary for many physiological and biochemical processes. Water is the most abundant
liquid on earth and it also simultaneously occurs on earth in solid and gaseous form. It is also almost the
only inorganic liquid and the others are not common (elemental mercury, for example).
The physical and chemical properties of water are responsible for the diverse forms and
interactions that it is capable of assuming. Water is a simple molecule composed of one oxygen atom
combined with two hydrogen atoms. In a sense it is oxidized hydrogen, the product of combustion of
organic and other materials. (Water is a product of cellular respiration, for example.) This simple
molecule normally would have characteristics similar to those of ammonia or hydrogen sulfide. That is, it
would almost exclusively occur in gaseous form at normal temperatures of the earth's surface.
However, its remarkable tendency to occur in liquid state is derived from the structure of the water
molecule. Oxygen has strong electronegative properties. Its combination with hydrogen results from
sharing electrons with the two hydrogen atoms. This is an example of covalent bonding with an inherent
assymetry to the bonds. The angle formed between the bonded hydrogen atoms is approximately 105
degrees. This angle is greater than theoretically predicted (90 degrees) because of the repulsive force
between the two similarly charged hydrogen atoms. The result is a polar molecule with the negative
charge on the oxygen end and the positive charge associated with the hydrogen. Water, then, is a polar
solvent. Furthermore, the electronegative pole associated with the oxygen of one molecule may form a
weak but significant bond with the electropositive or hydrogen portion of another water molecule, a
1.2-7
|
||
 |
||