
|
|

|
||
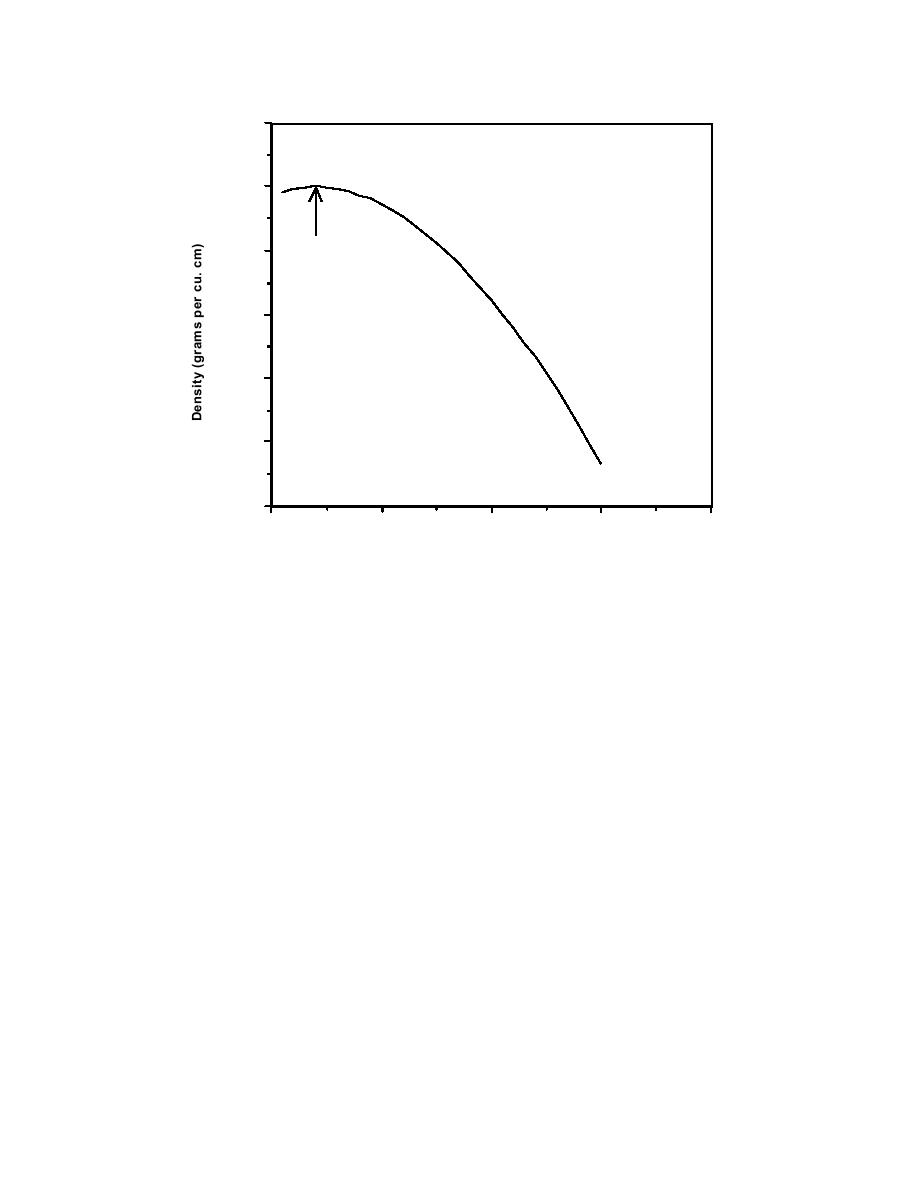 1.001
Relationship Between Temperature and Density
for Pure Water
1.000
3.96 C
0.999
0.998
0.997
0.996
0.995
0.0
10.0
20.0
30.0
40.0
Temperature (C)
Figure 1.2.3 The relationship between temperature and density for pure water.
This is true for most lakes. Exceptions exist where density is influenced by salinity
or other chemical concentrations.
At the other phase change, liquid into gas, energy is again absorbed at relatively constant
temperature until all liquid has been vaporized. As with the heat of fusion, the temperature at which this
process takes place is dependent on the pressure expressed by the environment. At standard pressure
this temperature is the boiling point, 100EC, and the energy associated with the conversion of water to
vapor (or the reverse) is the "latent heat of vaporization". In this case the amount of energy required for
the conversion of water to vapor is quite large, approximately 540 calories per gram of water. This is
important for the energy or thermal characteristics of lakes as will be explained later. In the atmosphere
this is also important to lakes because the conversion of atmospheric vapor to rain releases this energy.
The energy is then available, for example, for expression as large pressure differentials resulting in
winds, storms, or even hurricanes.
Between these temperature bounds, water has several interesting behaviors and characteristics.
The "specific heat" of water is 1 or unity, defined as 1 calorie of energy required to increase 1 gram of
water by 1EC. Density, as shown earlier, is dependent on temperature in a nonlinear manner. Density is
also greatly affected by dissolved substances and most persons are aware that salinity increases density
as well. If the maximum density of pure water is, by definition, 1 gram per cu.cm then any condition
1.2-9
|
||
 |
||