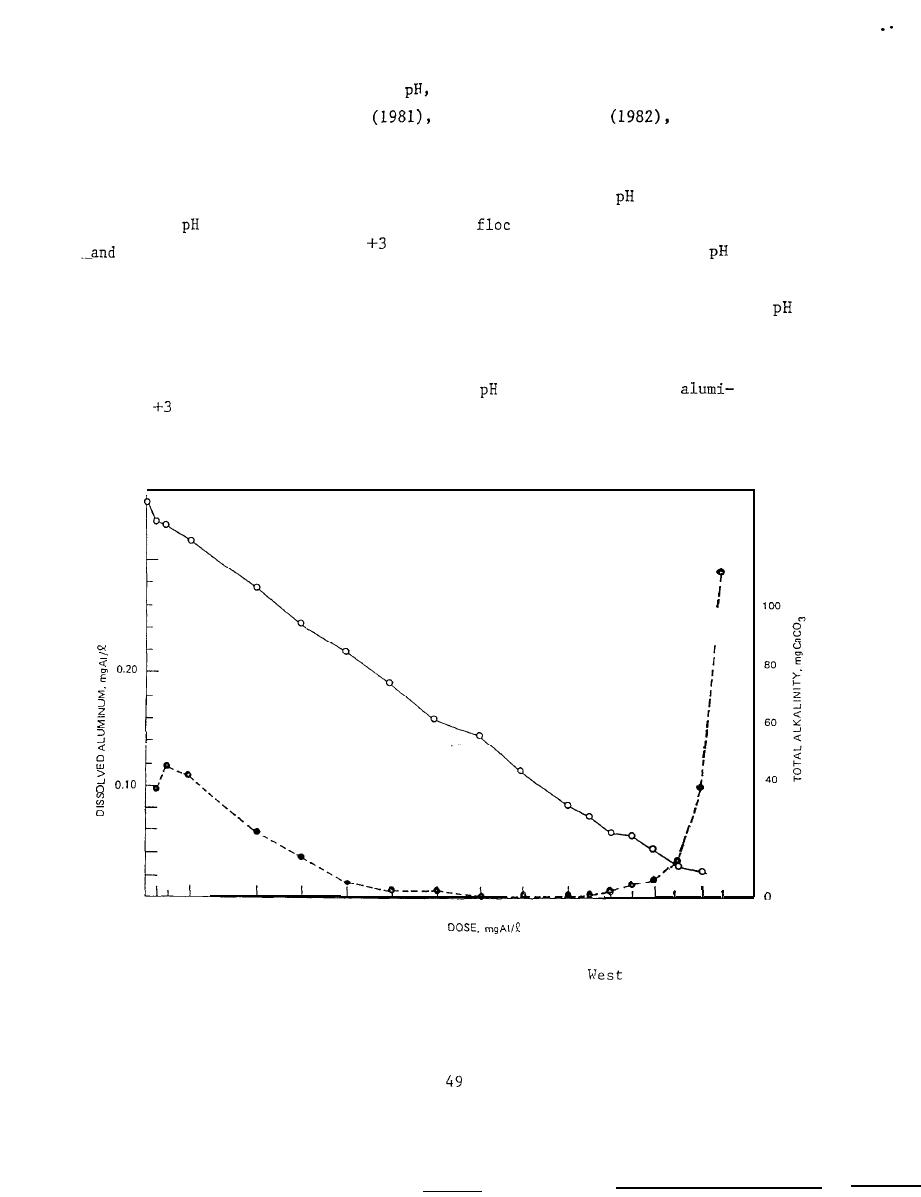
relationships between alkalinity,
and aluminum dose.
Kennedy's method is
reviewed in Cooke and Kennedy
Kennedy and Cooke
and Cooke
et al. (1986). The procedure, which is applicable for reservoirs also, is
briefly outlined here.
When aluminum sulfate is added to reservoir water,
and alkalinity
fall.
At
6 to 8, large amounts of the
aluminum hydroxide are formed,
the -dissolved aluminum (Al
) concentration remains low.. This
range is
therefore ideal since large amounts of phosphorus will be sorbed, and toxic
conditions will not be present.
However, with further additions of alum,
values below 6.0 occur and the concentration of dissolved aluminum increases
rapidly (Figure 6).
Therefore, the maximum amount of aluminum sulfate that
can be added before the appearance of low
and high dissolved
num (Al
) is dependent upon the initial alkalinity of the reservoir water.
The maximum dose is therefore unique to each reservoir.
General guidelines
140
120
0.08
0.06
20
0.04
0.02
0
2
5
7
0.5
9
11
13
15
17
19
21
23
25
ALUMINUM
Figure 6.
Changes in dissolved aluminum concentration (dashed line)
and total alkalinity (solid line) for water from
Twin Lake,
Ohio. treated with increasing doses of aluminum sulfate
(after Cooke et al. 1978)



 Previous Page
Previous Page
